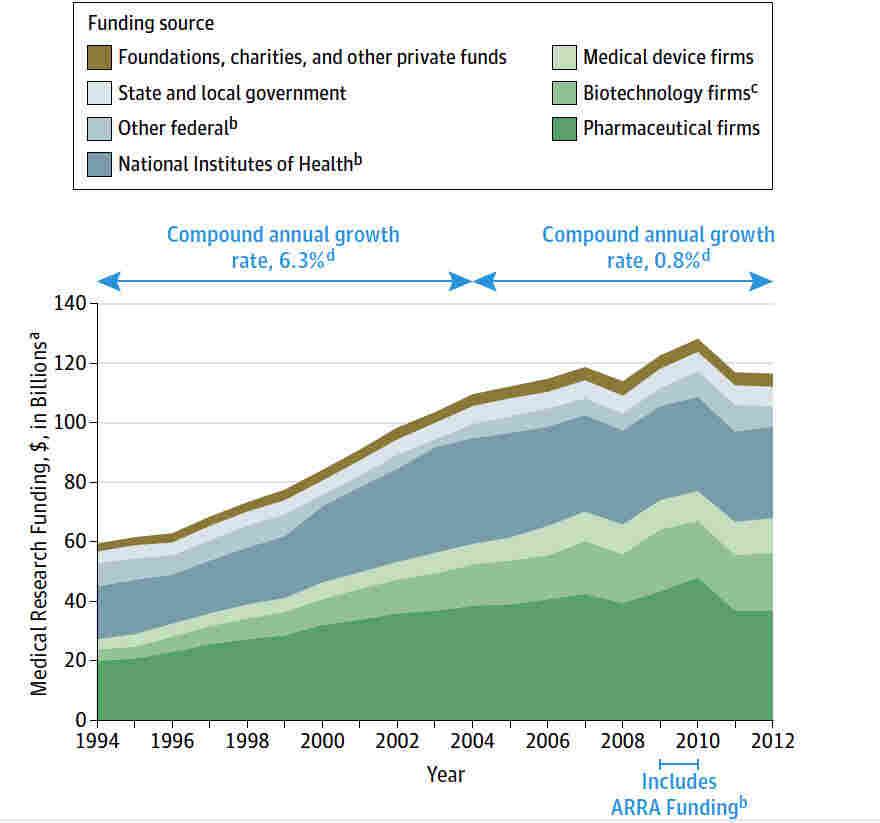
Medical research funding is crucial for advancing healthcare and ensuring patient safety in clinical trials. Recently, however, significant cuts to this funding have raised alarms about the potential negative impact on the comprehensive oversight provided by Institutional Review Boards (IRBs) during research. Such disruptions threaten the delicate balance necessary in the IRB review process, essential for safeguarding the rights and well-being of participants involved in clinical studies. For instance, the National Institutes of Health (NIH) funding importance cannot be overstated, as it directly correlates with the quality and scope of research endeavors aimed at improving therapeutic options. Collaborative research oversight has never been more vital, as we face the challenge of maintaining high ethical standards amid dwindling resources.
The financial backing of health research initiatives plays a pivotal role in shaping successful medical investigations and enhancing the well-being of individuals who participate in these studies. With an urgent demand for rigorous evaluations, funding allocations to research organizations influence both the execution of experiments and the comprehensive review necessary to protect volunteer participants. In this context, understanding the adverse effects of budget cuts on investigative work becomes paramount, especially concerning essential areas like ensuring patient safety and ethical standards. These challenges also highlight the critical need for continuous and sufficient support mechanisms, as institutions strive to navigate the complexities of multi-site collaboration and effective oversight in medical research.
Impact of Funding Cuts on Medical Research Oversight
The recent freeze of over $2 billion in federal research grants has sent shockwaves through the medical research landscape, severely affecting oversight mechanisms essential for maintaining patient safety. Such funding cuts not only disrupt ongoing studies but also hinder the Institutional Review Board (IRB) review process, which is crucial for protecting the rights of research participants. As resources dwindle, the capacity for thorough reviews diminishes, raising significant concerns about the adherence to ethical standards in multiple-site studies that require collaborative oversight.
Moreover, without adequate funding, many IRBs face challenges in their operational effectiveness. The SMART IRB system, designed to streamline the review process across various institutions, finds itself hampered by these financial constraints. The ongoing struggles directly impact the ability of researchers to initiate new clinical trials, subsequently risking delays in medical advancements that could improve patient care and safety. As studies pause or terminate, it not only affects the immediate research goals but also fosters skepticism within communities about the reliability and integrity of future medical research.
The Role of NIH Funding in Patient Protection
NIH funding plays a pivotal role in safeguarding patients involved in clinical trials by ensuring that research adheres to strict oversight and ethical guidelines laid out by the IRB process. Federal funds typically cover the indirect costs associated with compliance, including training programs for researchers and staff, which are essential for recognizing potential risks to participant safety. By underpinning the regulatory framework, NIH funding helps to sustain the diligence required for effective participant protection, which is paramount in any medical research endeavor.
Further, the significance of NIH funding extends to promoting a collaborative approach among institutions conducting similar research. With requirements for a single IRB review introduced for multisite studies, NIH funding allows these institutions to share resources and expertise efficiently, reducing delays that may arise from bureaucratic red tape. The lack of sufficient funding jeopardizes this collaboration, consequently leading to fragmented research efforts and undermining the progress that has been made to ensure participants’ safety and ethical standards.
Understanding the Importance of Institutional Review Boards (IRBs)
Institutional Review Boards (IRBs) are critical in the medical research ecosystem, acting as gatekeepers that protect the rights and welfare of participants. Their role involves thorough evaluations of research protocols to ensure that potential risks are mitigated, informed consent is achieved, and that ethical standards are upheld. As federal funding cuts threaten the resources available to IRBs, their capacity to fulfill these responsibilities may be compromised, potentially leading to lapses in patient safety during clinical trials.
Historically, IRBs emerged from a necessity to rectify past abuses in medical research, such as the infamous Tuskegee Syphilis Study. Today, the challenges that IRBs face are magnified by funding constraints, which can result in limited staff, inadequate training, and reduced capacity for monitoring ongoing studies. Consequently, this poses a risk not just to those currently participating in research but also risks undermining public trust in clinical trials and the broader research community as a whole.
Collaborative Research Oversight: A Necessity for Ethical Standards
Collaborative research oversight is essential for maintaining ethical standards across multiple research sites. With the introduction of single IRBs for multisite studies, researchers can expedite their efforts to gather data without compromising on the oversight necessary to protect participant safety. However, funding cuts threaten the viability of these collaborative models, reducing the resources available to coordinate and oversee complex research efforts effectively.
The consequences of inadequate oversight extend beyond individual studies, influencing public perceptions of research integrity and patient safety. When funding is restricted, institutions may struggle to maintain rigorous review processes or support extensive monitoring of research activities, which could lead to ethical breaches. Therefore, adequate funding is crucial for fostering collaboration across institutions, enabling them to adhere to high standards in patient safety, and ensuring that ethical research practices are upheld.
The Effects of Federal Funding Cuts on Patient Safety
Federal funding cuts directly jeopardize the safety of patients involved in medical research. A halt in vital financial resources disrupts not only ongoing clinical trials but also the comprehensive oversight mechanisms that are crucial for ensuring participant welfare. Resources dedicated to participant education, risk assessment, and consent processes can dwindle, leading to potentially unsafe clinical practices if caution is not maintained.
Moreover, a lack of funding can hinder the ability of research teams to implement necessary safety protocols and respond promptly to adverse events. With fewer funds available to conduct regular audits and evaluations, institutions may face growing difficulty in ensuring compliance with both institutional and federal regulations. This lack of oversight can foster an environment where patient safety is put at risk, creating broader public health concerns as researchers navigate these challenges.
How Financial Constraints Affect Innovative Research in Healthcare
Financial constraints can stifle innovation in healthcare research as institutions grapple with limited resources to explore new treatment avenues and methodologies. The failure to adequately fund groundbreaking studies hampers the potential for discovering life-saving therapies and technologies that address critical health issues. Research teams are often forced to prioritize projects based on available funding rather than the potential impact of the research on patient outcomes.
Furthermore, as more research proposals face rejection due to funding limitations, seasoned scientists may be deterred from entering fields that require extensive collaboration and rigorous oversight. The loss of intellectual capital is particularly detrimental to healthcare as it diminishes the pool of experts who can contribute to patient-centered research initiatives. To foster genuine progress, there is an urgent need for renewed investment in medical research funding, ensuring that innovative solutions continue to emerge for today’s healthcare challenges.
Medical Research Funding: A Pillar of Success
Medical research funding serves as a pillar for successful patient outcomes and ethical research practices. With substantial federal and institutional backing, researchers are equipped with the resources necessary to conduct thorough investigations, including extensive IRB reviews and compliance checks. These funds not only allow for basic research but also enable advanced studies that focus on translating laboratory findings into effective treatments and preventive measures.
However, when funding is threatened, the risk extends beyond immediate financial implications. A reduction in medical research funding can impede future innovations and discourage ambitious research programs focused on chronic diseases and public health crises. Ensuring robust funding for research initiatives is vital for sustaining the momentum of medical advancements and protecting the health and safety of patients across diverse populations.
Maintaining Patient Trust in Medical Research
Building and maintaining patient trust in medical research is paramount, particularly in light of funding cuts that may raise concerns about ethical standards and oversight. Patients who participate in clinical trials deserve assurance that their safety is the top priority, which is upheld through rigorous adherence to IRB guidelines and ethical practices. As federal support wanes, establishing a clear and transparent operating environment becomes even more critical to instill confidence among potential research participants.
Moreover, when funding interruptions compromise the integrity of studies, it can lead to skepticism in the community regarding the motives behind research endeavors. Educating the public about the mechanisms in place, such as user-friendly IRB processes and robust participant protections, can help alleviate fears. By actively engaging with communities and promoting openness about research methodologies, institutions can foster trust and encourage active participation in legitimate medical research initiatives.
The Future of Medical Research amidst Funding Challenges
The future of medical research may hinge on overcoming current funding challenges while preserving ethical standards and protecting patient safety. Innovation and discovery depend heavily on sustained investment from both federal sources like NIH and private-sector partnership funding. Researchers must advocate for the importance of robust funding streams to enable comprehensive review processes and effective monitoring of studies that prioritize patient welfare.
The commitment to preserving the integrity of medical research amidst these challenges will shape the landscape of clinical trials moving forward. As funding ebbs and flows, so too does the potential for transformative advancements in health. Addressing these funding issues head-on is critical to ensuring that researchers can continue their work responsibly and ethically, ultimately leading to better health outcomes for countless individuals.
Frequently Asked Questions
What is the impact of funding cuts on medical research and patient safety?
Funding cuts can severely hinder medical research efforts, particularly those aimed at patient safety. When grants are frozen or canceled, it disrupts ongoing studies and compromises the institutional review board (IRB) process that ensures participant safety in clinical trials. This can lead to delays and a lack of necessary oversight, ultimately risking the health and trust of patients involved in research.
How does NIH funding contribute to ensuring patient safety in clinical trials?
NIH funding is crucial for ensuring patient safety in clinical trials as it supports the infrastructure necessary for rigorous IRB reviews and compliance with ethical standards. This funding allows institutions to employ trained professionals who oversee study protocols, informed consent processes, and monitor participant safety throughout the research.
What role does the IRB review process play in medical research funding?
The IRB review process is fundamental in the realm of medical research funding, particularly because it safeguards the rights and welfare of participants. Many federally funded studies, especially those supported by NIH, require an IRB review to assess and mitigate risks, ensuring that research efforts adhere to ethical guidelines.
Why is NIH funding importance critical for collaborative research oversight?
NIH funding plays a critical role in collaborative research oversight by providing resources for a centralized IRB (sIRB) system. This streamlines the review process across multiple sites and enhances collaborative efforts, thereby enabling researchers to conduct studies more efficiently while maintaining high standards for participant safety.
How do funding cuts affect collaborative research efforts in medical studies?
Funding cuts can significantly disrupt collaborative research efforts by halting studies that require joint oversight from multiple institutions. A stop-work order on federal research contracts prevents new sites from participating, limiting the ability to conduct comprehensive research that ensures patient safety, ultimately stalling progress in medical advancements.
| Key Point | Details |
|---|---|
| Impact of Funding Cuts | The Trump administration’s halt of over $2 billion in federal research grants disrupts patient safety and rights. |
| Function of IRBs | Institutional Review Boards (IRBs) protect patients by overseeing research, ensuring compliance with laws, and safeguarding the welfare of participants. |
| Importance of SMART IRB | The SMART IRB system simplifies collaboration across institutions, critical for efficient research processes. |
| Historical Context | IRBs were established in response to historical abuses in medical research, emphasizing ethics and oversight. |
| Broader Implications of Cuts | Ongoing funding cuts can undermine public trust in research and lead to significant delays in critical studies. |
Summary
Medical research funding is crucial to ensuring patient safety and the integrity of clinical trials. The recent halt in funding has disrupted essential oversight and oversight mechanisms, particularly affecting institutional review boards pivotal for protecting human participants in research. Without sufficient financial support, efforts to safeguard rights and welfare in medical studies are seriously compromised, posing risks not only to participants but also to the advancement of medical science itself. Effective and ethical research is dependent on consistent and adequate funding.