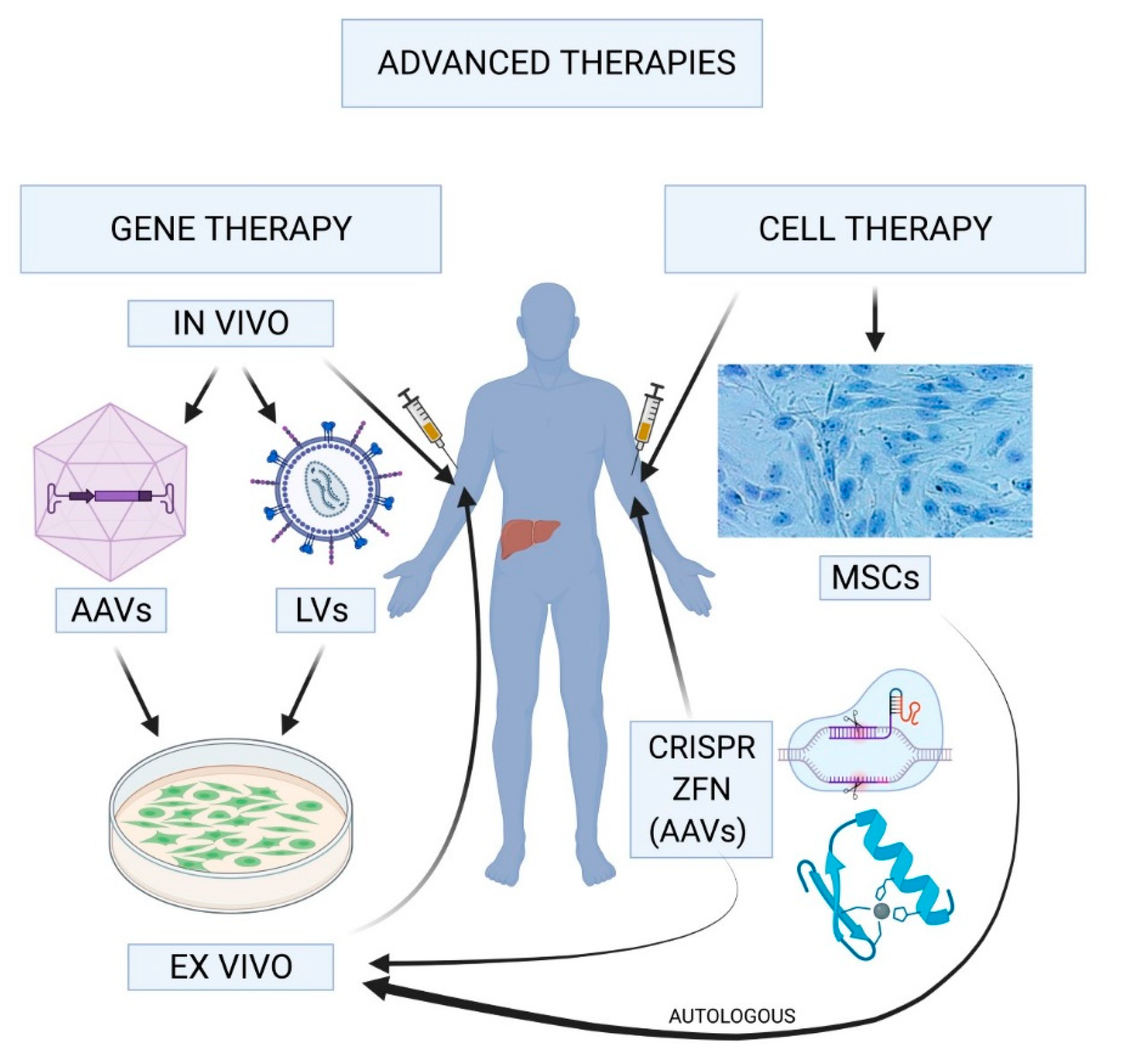
Gene therapy for hemophilia B represents a groundbreaking advancement in hemophilia treatment that could reshape the lives of those affected by this disorder. Utilizing revolutionary techniques, such as Hemgenix, this innovative therapy offers the potential to correct the genetic abnormalities responsible for hemophilia, reducing the dependency on traditional clotting factor therapy. Patients like Terence Blue have experienced the remarkable benefits of gene therapy, which not only alleviates the burden of daily injections but also promises a healthier, more autonomous life. With a growing focus on new hemophilia therapies, the landscape of treatment modalities is rapidly evolving, offering hope to thousands living with this condition. As research continues to expand, the anticipation surrounding gene therapy benefits grows, marking an important era in the management of hemophilia B.
The recent developments in hemophilia treatment underscore the significance of gene therapy as an emerging solution for hemophilia B, a condition characterized by inadequate production of clotting factors. Alternative terms such as genetic modification and precision medicine highlight the advanced methodologies being employed to address this genetic disorder. Hemgenix, a notable example, showcases how targeted therapies can alter the course of hemophilia management, moving beyond conventional clotting factor replacement. This shift towards innovative approaches emphasizes the potential of new hemophilia therapies to provide not just symptom management but a transformative solution for affected individuals. As we explore the intricacies of these therapies, the conversation on their advantages continues to gain momentum in the medical community.
Understanding Hemophilia B: A Patient’s Journey
Hemophilia B, a genetic disorder characterized by the deficiency of clotting factor IX, has long posed significant challenges for those affected. Patients like Terence Blue navigate a life punctuated by the challenge of managing their condition, which often requires frequent treatments to prevent excessive bleeding. This journey can be daunting, as it not only affects physical health but also mental well-being. For many, the constant need for clotting factor therapy becomes an unyielding reminder of their condition, compounded by the social stigma and misconceptions that often surround it.
Blue’s experience highlights the importance of proactive management in hemophilia B. Intravenous injections of clotting factor have traditionally been the mainstay of treatment. However, the emergence of innovative therapies, including gene therapy, presents new hope. For patients, understanding the evolution of these treatments and the potential benefits they bring is crucial. Today’s treatments, such as Hemgenix, aim to address not only the symptoms but the underlying genetic cause, providing a glimpse of a future where living free of frequent interventions might be a reality.
The Role of Gene Therapy in Transforming Hemophilia Treatment
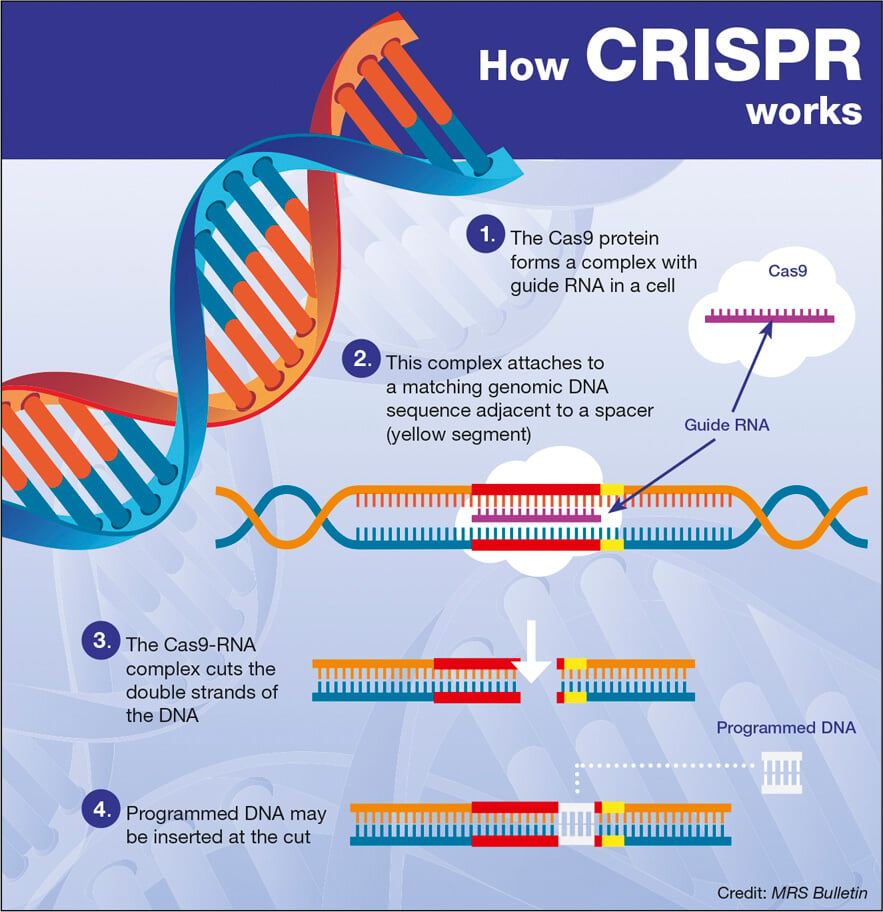
Gene therapy represents a revolutionary advancement in the treatment of hemophilia B, offering patients the prospect of long-term relief from the burdens of regular clotting factor treatments. Terence Blue’s path to receiving Hemgenix therapy underscores the unique potential of gene therapy to change lives. During the administration of Hemgenix, the therapy works to alter the genetic information within liver cells, enabling the body to produce its own clotting factor IX, reducing or eliminating the need for ongoing treatment. This leap forward in hemophilia treatment not only aims to improve patients’ quality of life but also lowers the long-term healthcare burden associated with frequent therapies.
The benefits of gene therapy extend beyond mere convenience; they encapsulate a transformative approach to hemophilia management. As Blue himself articulated, having the opportunity to live with reduced anxiety over bleeding episodes while avoiding a lifetime of needles is a significant milestone. Moreover, data indicates that most patients treated with Hemgenix do not require additional factor IX prophylaxis for several years after treatment, positioning gene therapy as a suitable long-term solution for many.
Exploring the Benefits of Hemgenix Gene Therapy
Hemgenix gene therapy, developed by CSL Behring, represents a beacon of hope for patients grappling with hemophilia B. Approved by the FDA in November 2022, this innovative treatment aims to insert a corrected copy of the factor IX gene directly into patients’ liver cells, ultimately leading to autonomous production of the critical clotting factor. This approach not only offers the advantage of a single-dose treatment but also brings significant long-term benefits, including reduced reliance on continuous infusion therapies that have traditionally defined hemophilia management.
The success of Hemgenix could pave the way for a new era in hemophilia treatment, moving from a model of chronic management towards potential cures. Patients like Terence Blue reflect on the relief that accompanies this approach, emphasizing both physical healing and an escape from the psychological burdens imposed by constant treatment. As gene therapy continues to evolve, it solidifies its place as a transformative option, promising a future where individuals with hemophilia can lead more normal lives with fewer medical interruptions.
Current Trends in New Hemophilia Therapies
The landscape of hemophilia treatment is rapidly changing, with a roster of new therapies emerging to provide better options for patients. Alongside gene therapies like Hemgenix, there is ongoing research and development of novel medications aimed at enhancing the safety and efficacy profiles of existing treatments. These innovations stem from a deep understanding of the genetic and molecular foundations of hemophilia, enabling scientists to create targeted therapies that can dramatically improve patient outcomes and quality of life.
As new hemophilia therapies continue to enter the pipeline, patients now have access to an array of choices that can address their specific needs. Options ranging from long-acting clotting factors to emerging gene therapies signify a shift in focus towards individualized treatment plans that prioritize patient preferences and lifestyle. This patient-centered approach, coupled with increased awareness and acceptance of novel therapies, is fostering an environment where living with hemophilia can be significantly improved.
Navigating Market Pressures in Gene Therapy Development
The path to bringing new hemophilia therapies like gene therapy to patients is not without its challenges. Despite the groundbreaking potential of treatments like Hemgenix, market pressures play a pivotal role in shaping the availability and affordability of these therapies. As seen with the withdrawal of Pfizer’s Beqvez for hemophilia B, the economics of gene therapy can complicate access, as high development costs and subsequent pricing strategies may limit patient uptake. Continued dialogue between stakeholders—including researchers, clinicians, and payers—is essential to create a sustainable model that supports both innovation and patient access.
While gene therapy promises long-lasting benefits for patients, it also presents economic challenges. Unlike conventional medications that provide ongoing revenue streams for pharmaceutical companies, gene therapies are typically administered as a one-time treatment. This difference complicates financial viability, leading to scrutiny over costs and returns on investment. Addressing these market pressures will require collaborative efforts to establish effective pricing models that ensure patient access while also supporting the significant R&D investments necessary for bringing such transformative therapies to market.
Overcoming Misconceptions Surrounding Hemophilia Treatments
Despite advancements in hemophilia treatments, misconceptions persist that can hinder patient acceptance of newer therapies such as gene therapy. Many individuals may associate hemophilia solely with traditional clotting factor therapies, failing to recognize the progress being made in developing alternative options. Oral education and outreach efforts play a crucial role in amplifying awareness of innovative treatments, like Hemgenix, that offer transformative possibilities. For patients, being informed about their options empowers them to make decisions that best align with their lifestyles and healthcare goals.
Moreover, the belief that gene therapies are experimental or not fully understood can lead to hesitance among patients and healthcare providers alike. It is crucial to acknowledge that rigorous clinical trials and FDA approvals are essential steps that ensure new treatments are safe and effective. Instilling confidence in the science behind gene therapy can help dispel fears and encourage individuals with hemophilia to consider options that could significantly reduce the burden of their condition.
The Impact of Clotting Factor Therapy on Patient Lives
Clotting factor therapy has been a cornerstone of hemophilia management for decades, fundamentally changing the lives of individuals living with this condition. By providing the missing clotting factor IX, these interventions prevent spontaneous bleeding episodes and allow patients like Terence Blue to engage in daily activities with greater confidence. As patients adhere to their treatment regimens, they often report improved quality of life and the ability to participate in physical activities that were previously deemed too risky.
However, the life-long commitment to regular injections can impose significant emotional and social burdens. Patients often navigate complexities when balancing their treatment needs with their personal and professional lives. The introduction of gene therapy options reflects a shift in addressing not just the physical symptoms of hemophilia but also the psychosocial aspects, offering hope for a future where individuals can enjoy greater freedom and less worry about managing their condition.
The Future of Hemophilia Care: A Patient-Centric Approach
The future of hemophilia care is increasingly leaning towards a patient-centric approach, where individual preferences and experiences drive the development and implementation of treatment options. As seen with the rollout of gene therapies like Hemgenix, the focus is shifting away from one-size-fits-all models, instead prioritizing personalized care that aligns with the diverse lifestyles of patients. This evolution is essential for empowering patients to take charge of their health, ensuring that therapies not only address the clinical aspects but resonate personally with each individual.
As researchers and healthcare providers continue to innovate, the importance of collaboration between patients, clinicians, and drug developers becomes apparent. Listening to patient feedback and experiences is crucial for shaping new treatments and improving existing ones. This inclusive approach will forge a future where individuals with hemophilia can expect not only effective medical solutions but also comprehensive support that fosters their overall well-being.
Real-Life Experiences: Patient Testimonials on New Therapies
Real-life experiences and testimonials from patients undergoing new hemophilia therapies can provide invaluable insights into the impacts of these advancements. Terence Blue’s personal account of receiving Hemgenix serves as a beacon of hope for others in the same situation, illustrating the transformative potential of modern treatment options. These narratives highlight not only the physical restoration that gene therapy can achieve but also the emotional relief of living without constant fear of bleeding episodes.
Sharing patient stories fosters a sense of community among those living with hemophilia, allowing individuals to connect over shared experiences and challenges. It reinforces the importance of support systems and advocacy within the hemophilia community, encouraging open dialogues about emerging therapies. As patients articulate their journeys, they inspire others to embrace new options and advocate for essential advancements in hemophilia care.
Frequently Asked Questions
What is gene therapy for hemophilia B and how does it work?
Gene therapy for hemophilia B, such as the FDA-approved Hemgenix, involves using a viral vector to deliver a corrected copy of the gene responsible for producing clotting factor IX into the liver. This enables the body to produce the missing clotting factor, greatly reducing or eliminating the need for traditional clotting factor therapy.
What are the benefits of gene therapy for hemophilia treatment?
The primary benefits of gene therapy for hemophilia treatment include the potential for long-term relief from the disease, reduced dependence on regular clotting factor injections, and improved quality of life. Patients like Terence Blue have experienced significant increases in clotting factor levels and reduced bleeding episodes after receiving gene therapy.
How effective is Hemgenix gene therapy in treating hemophilia B?
Hemgenix has shown promising results in clinical trials, with about 94% of patients not requiring factor IX prophylaxis three years post-treatment. This indicates long-lasting effects and the potential to provide significant improvement in managing hemophilia B.
Will gene therapy replace traditional clotting factor therapies for hemophilia B?
While gene therapy for hemophilia B offers significant advancements, it may not completely replace traditional clotting factor therapies for all patients, particularly those who may have specific medical considerations. However, gene therapy presents a revolutionary option for many seeking new hemophilia therapies.
What are the side effects associated with gene therapy for hemophilia B?
Patients receiving gene therapy for hemophilia B, such as Hemgenix, may experience some side effects, including elevated liver enzymes or reactions to the infusion process. Close monitoring by healthcare providers ensures any issues are promptly addressed.
How is gene therapy for hemophilia B administered?
Gene therapy for hemophilia B is typically administered as a one-time infusion. In the case of Hemgenix, the treatment is delivered via IV into the patient’s bloodstream, targeting the liver to facilitate gene transfer and subsequent production of clotting factor IX.
Are there any age restrictions for receiving gene therapy for hemophilia B?
Gene therapy for hemophilia B, including Hemgenix, may have specific age guidelines based on clinical trial data and FDA recommendations. Consult with a healthcare provider to determine if a patient meets eligibility criteria.
What is the cost of gene therapy for hemophilia B and is it covered by insurance?
The cost of gene therapy for hemophilia B, such as Hemgenix, is approximately $3.5 million. While insurance providers may negotiate lower rates, coverage can vary, making it essential to check with your insurance for details on potential costs and coverage.
Can gene therapy provide a cure for hemophilia B?
While gene therapy offers the potential for long-lasting effects and significantly improved health, it is typically described as a treatment rather than a definitive cure. Many patients experience substantial reductions in symptoms, leading to a much-improved quality of life.
What is the future of gene therapy for hemophilia and other bleeding disorders?
The future of gene therapy for hemophilia and other bleeding disorders looks promising, with ongoing research and development aiming to improve safety and efficacy. As more gene therapies are approved, patients can expect expanded options that may transform their treatment experience.
| Point | Details |
|---|---|
| Patient Background | Terence Blue, diagnosed with hemophilia at birth, managed his condition for 27 years with frequent hospital visits and injections. |
| Introduction of Gene Therapy | Blue became the first patient in New England to receive Hemgenix, a new gene therapy for hemophilia B, which offers the prospect of reduced reliance on factor IX injections. |
| Mechanism of Action | Hemgenix utilizes a virus to deliver a corrected gene into liver cells, enhancing the production of the missing clotting factor IX. |
| Economic Considerations | Gene therapies are significantly expensive, with Hemgenix listed at $3.5 million, posing challenges to market accessibility. |
| Long-term Efficacy | Clinical trials show 94% of participants do not require factor IX prophylaxis three years post-treatment. |
| Patient Experience | Blue experienced notable health improvements, reporting faster healing and reduced need for factor IX. |
Summary
Gene therapy for hemophilia B represents a groundbreaking advancement in the treatment of this chronic bleeding disorder. With treatments like Hemgenix offering the potential for long-term relief and significantly improved quality of life, patients can look forward to a future with less dependence on frequent injections of clotting factors. Terence Blue’s journey exemplifies the transformative potential of gene therapy, which not only addresses the underlying genetic causes but also hints at a new era of personalized medicine, where the burdens of hemophilia can be minimized or even eradicated.