TIM-3 Alzheimer’s therapy is an innovative approach that harnesses the body’s immune system to tackle the challenges posed by Alzheimer’s disease. Recent research has illuminated the potential of the TIM-3 molecule in promoting cognitive function improvement by freeing brain immune cells, known as microglia, to effectively target and clear harmful plaques from the brain. This development draws parallels to established cancer treatment strategies, where immune checkpoint molecules have been manipulated to enhance anti-tumor responses. The study highlights how silencing TIM-3’s inhibitory effects can invigorate microglial activity, ultimately aiming for breakthroughs in Alzheimer’s disease treatment. With these findings paving the way for novel therapies, there is a growing optimism about the role of TIM-3 in combating the cognitive decline associated with Alzheimer’s.
The therapeutic landscape for Alzheimer’s disease is evolving, particularly with promising alternatives like TIM-3 inhibition. This cutting-edge strategy looks to leverage the immune response to improve brain health, highlighting the critical role of immune cells in managing plaque accumulation. By silencing the TIM-3 molecule, researchers are investigating novel avenues for enhancing the functionality of microglia, the brain’s primary immune defenders. This could lead to significant advancements in Alzheimer’s management, mirroring immune-related strategies that have shown success in cancer treatments. As the scientific community delves deeper into this promising research, the hope for effective Alzheimer’s disease treatments grows stronger.
Understanding TIM-3 and Its Role in Alzheimer’s Disease
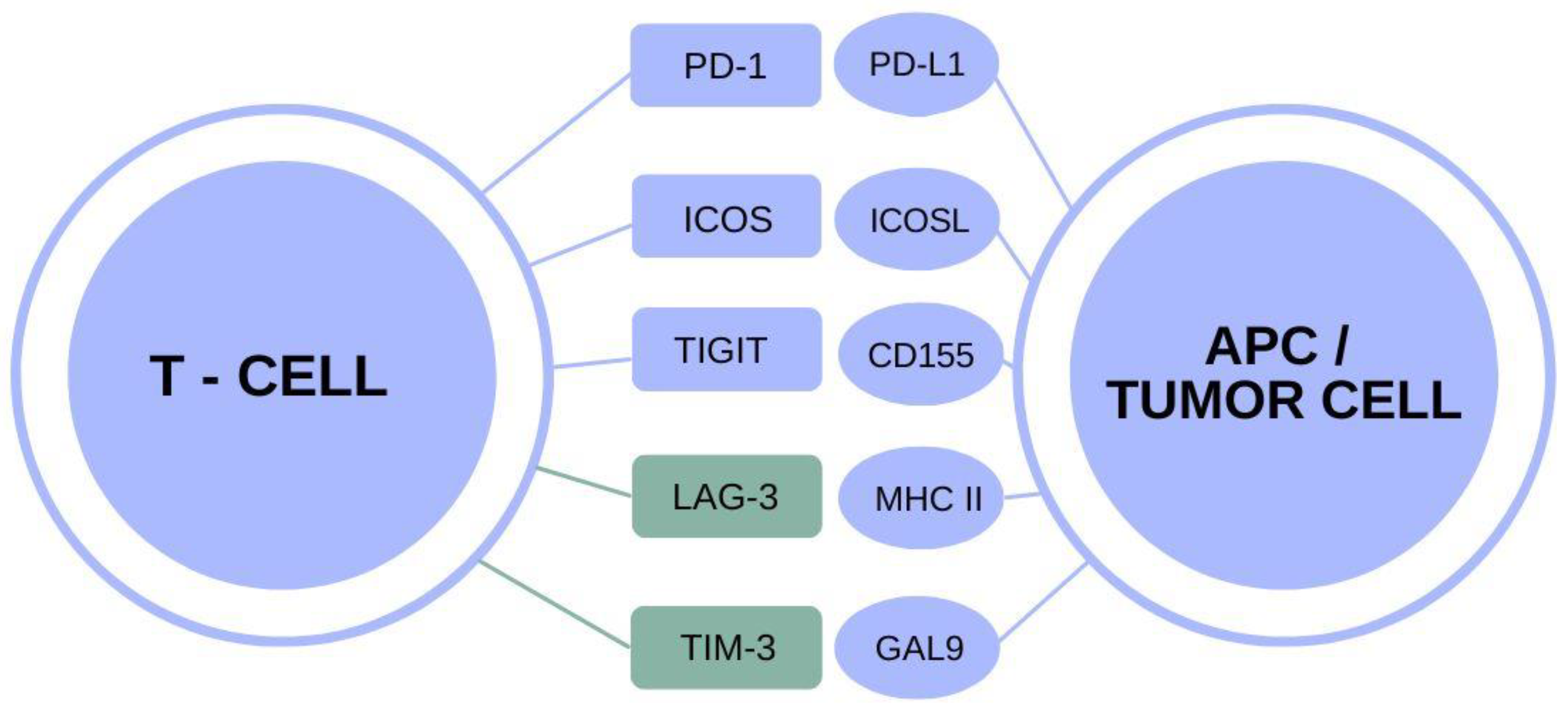
The TIM-3 molecule plays a pivotal role in the modulation of immune responses in the brain, particularly concerning Alzheimer’s disease. This inhibitory molecule, primarily found on microglial cells, is crucial for maintaining homeostasis within the central nervous system. However, its overexpression in aging individuals and Alzheimer’s patients has been linked to a reduced ability of these immune cells to clear amyloid plaques, which are hallmark features of the disease. This connection underscores TIM-3 as not just a regulatory molecule, but a significant player in the pathology of Alzheimer’s, presenting it as a potential target for therapeutic interventions.
Recent studies have employed genetically modified mice lacking the TIM-3 gene to explore the molecule’s impact on cognitive function and plaque clearance. In these models, researchers observed enhanced plaque removal by microglia, leading to improved memory and cognitive behavior. This evidence reinforces the idea that inhibiting TIM-3 function could open new avenues for Alzheimer’s disease treatment, suggesting that therapies aimed at blocking TIM-3 might rejuvenate microglial activity and restore cognitive functions.
The Mechanism of Cognitive Function Improvement in Alzheimer’s Therapy
One of the most compelling findings in the investigation of TIM-3 is its role in cognitive function improvement through the modulation of microglial activity. When TIM-3 expression is reduced or silenced, microglia are revitalized to engage more effectively with amyloid plaques, which are toxic aggregates that accumulate in the brains of Alzheimer’s patients. By clearing these plaques, microglia help restore neural health, leading to notable improvements in cognitive abilities such as memory and learning. This mechanism illustrates how therapeutic strategies targeting the TIM-3 pathway can convert dormant immune responses into active clearance operations, making them valuable for Alzheimer’s treatment.
Therapeutic applications could include the development of anti-TIM-3 antibodies or small molecules designed to inhibit TIM-3’s inhibitory function on microglia. By unlocking the potential of these immune cells, researchers hope to harness the brain’s innate cleaning mechanisms, thereby promoting healthier brain environments conducive to cognitive function. If successful, such strategies could represent a paradigm shift in Alzheimer’s care, moving from conventional amyloid targeting to improving overall brain health through immune system engagement.
Exploring Cancer Treatment Strategies in Alzheimer’s Research
The intriguing overlap between cancer treatment strategies and Alzheimer’s research highlights the adaptability of therapeutic approaches across diseases. Checkpoint inhibitors, which have revolutionized cancer care by blocking molecules like TIM-3, could also be engineered to benefit Alzheimer’s patients. Since TIM-3 inhibits immune cell activation, strategies that inhibit its function might encourage microglia to target amyloid plaques more aggressively, similar to how T cells attack tumor cells in cancer. This cross-disciplinary approach illustrates the potential for innovations in cancer treatment to inform new therapies for neurodegenerative diseases.
By leveraging insights gleaned from the mechanisms of immune suppression in cancer, researchers are beginning to appreciate how these strategies can be reinterpreted for neurodegenerative conditions. In Alzheimer’s, the myriad interactions between immune cells and neuronal health provide fertile ground for novel therapies. Furthermore, understanding how immune regulation operates in cancer and expanding that knowledge to Alzheimer’s could lead to significant breakthroughs in enhancing the quality of life for patients suffering from these debilitating diseases.
The Role of Microglial Cells in Alzheimer’s Pathology
Microglial cells are the brain’s resident immune cells and serve critical functions beyond their immune roles. In the context of Alzheimer’s disease, their ability to manage plaque clearance is impeded by the overexpression of checkpoint molecules like TIM-3. In healthy brains, microglia actively remodel synapses and eliminate damaged neurons, but in Alzheimer’s patients, the inability to effectively respond to amyloid plaques becomes detrimental. This makes the TIM-3 pathway a compelling target for restoring the normal function of microglial cells.
Research indicates that when microglial TIM-3 is silenced, these immune cells can resume their role in cleaning up amyloid plaques, potentially reversing cognitive decline. This underscores the necessity of understanding not just the pathology of Alzheimer’s, but also how microglial dynamics interact with immune checkpoint regulation. By navigating this complex relationship, researchers aim to develop therapeutic interventions that empower microglia to overcome the obstacles posed by amyloid beta accumulation, ultimately improving cognitive health.
Potential Therapeutic Strategies Targeting TIM-3 in Alzheimer’s Disease
The prospect of a TIM-3-based therapy for Alzheimer’s disease introduces exciting possibilities for ameliorating cognitive decline. Utilizing anti-TIM-3 antibodies or inhibitors to block the molecule’s function could reset the activity of microglia, enabling them to combat amyloid plaques effectively. This therapeutic strategy draws on established cancer treatments that utilize checkpoint inhibitors to unleash T-cell activity, transforming them into crucial players in immunotherapy. Applying similar principles to Alzheimer’s therapy proposes a novel approach to manage the disease via immune modulation.
Moreover, clinical trials based on TIM-3 inhibition may usher in a new era of Alzheimer’s treatment characterized by enhanced cognitive giving new hope to patients and families impacted by this relentless disease. The concept of transforming microglial responses through TIM-3 blockade bears significant relevance, suggesting that comprehensive immune system engagement might hold the key to not only slowing the progression of Alzheimer’s but potentially reversing some of its damaging effects.
Genomic Insights and TIM-3’s Link to Alzheimer’s Disease
Genomic studies have illuminated the genetic underpinnings of Alzheimer’s disease, notably implicating the TIM-3 gene (HAVCR2) as a significant risk factor for late-onset forms of the condition. This connection elucidates the molecular mechanisms that govern Alzheimer’s progression and highlights the importance of TIM-3 in maintaining a delicate balance between immune activation and inhibition. Understanding the polymorphisms associated with TIM-3 expression can provide insights into patient risk profiles, leading to personalized therapeutic strategies that consider genetic predisposition.
By incorporating genomic data into clinical practice, researchers can develop targeted therapies that exploit TIM-3’s role in the immune response. For instance, patients exhibiting higher levels of TIM-3 might benefit from interventions specifically designed to inhibit this molecule, thus enhancing the capacity of microglia to clear amyloid aggregates. Such nuanced approaches maximize treatment effectiveness by aligning with individual genetic backgrounds, ultimately striving for improved outcomes in managing Alzheimer’s disease.
Future Directions in TIM-3 Research for Alzheimer’s Treatment
The future of TIM-3 research holds promise for unlocking novel therapeutic avenues in Alzheimer’s disease management. As scientists seek to create humanized models with integrated TIM-3 genes, they are paving the way for precise evaluations of candidate therapies and their impacts on plaque accumulation and cognitive recovery. These innovative approaches inherently emphasize the necessity of interdisciplinary collaboration, drawing on advancements from immunology, neurology, and genetics to foster comprehensive treatment models.
Moreover, targeted therapies based on TIM-3 inhibition could be swiftly translated into clinical trials, provided that foundational research continues to yield promising results. In refining methods to modulate microglial function through TIM-3, researchers are harnessing the brain’s immune system to combat neurodegeneration, thereby reshaping the therapeutic landscape for Alzheimer’s disease. Ultimately, continual investment in TIM-3 research could lead to groundbreaking strategies that enhance cognitive function and improve life quality for millions.
Microglia in Aging and Alzheimer’s Disease: The TIM-3 Connection
As individuals age, the activity of microglial cells undergoes significant changes, which can have profound implications for Alzheimer’s disease. Under normal circumstances, microglia play a pivotal role in synaptic pruning and neural maintenance; however, the expression of TIM-3 increases with age, leading to a homeostatic state where these cells are less effective at clearing harmful amyloid plaques. Understanding this transition in microglial behavior as a direct consequence of aging and TIM-3 expression is vital for developing age-appropriate therapies that can reactivate these cells’ functions.
Recent studies illustrate that modulation of TIM-3 can rejuvenate the microglial response even in aged models, emphasizing the potential for therapeutic strategies that reset cognitive function. By alleviating the detrimental effects of TIM-3 on microglial activity, researchers aim to target one of the primary impediments to successful Alzheimer’s treatments. Through continued exploration of this connection between aging, microglial health, and TIM-3 function, the scientific community may derive actionable insights that transform the therapeutic approach for age-related neurodegenerative diseases.
The Interplay Between Inflammation, Amyloid Plaques, and TIM-3 in Alzheimer’s
The interplay between inflammation and amyloid plaque accumulation in Alzheimer’s disease is intricately linked to the actions of TIM-3. Chronic inflammation is a hallmark of Alzheimer’s pathology and is exacerbated by the presence of amyloid beta plaques. TIM-3 acts as a checkpoint in this inflammatory response, suppressing microglial activity and their ability to clear plaques. As a result, therapeutic approaches aimed at modulating TIM-3 activity seek to unleash microglial responses to inflammation and facilitate the removal of toxic debris.
Understanding how TIM-3 mediates inflammation in the context of Alzheimer’s not only positions it as a target for novel therapies but also highlights the complex relationship between immune activation and neurodegeneration. By devising strategies that restore proper immune function via TIM-3 inhibition, researchers aim to create an environment conducive to effective plaque clearance and cognitive preservation. As investigations continue, the goal remains clear: to translate basic research into therapies that can genuinely mitigate the impact of Alzheimer’s disease on the lives of affected individuals.
Frequently Asked Questions
What is TIM-3 Alzheimer’s therapy?
TIM-3 Alzheimer’s therapy refers to an innovative treatment strategy targeting the TIM-3 molecule, known to inhibit microglia, which are critical immune cells in the brain. Research suggests that silencing TIM-3 can enhance microglial activity, enabling them to clear amyloid plaques associated with Alzheimer’s disease, thereby potentially improving cognitive functions.
How does the TIM-3 molecule affect Alzheimer’s disease treatment?
The TIM-3 molecule plays a significant role in Alzheimer’s disease treatment by inhibiting microglia from attacking amyloid plaques. By targeting TIM-3, researchers aim to release these immune cells from suppression, allowing them to effectively clear harmful plaques and improve memory and cognitive functions in patients with Alzheimer’s.
Can microglia immune cells be activated to improve cognition in Alzheimer’s patients?
Yes, microglia immune cells can be activated for cognitive improvement in Alzheimer’s patients. Research on TIM-3 indicates that disabling this inhibitory checkpoint molecule helps microglia engage plaque clearance mechanisms, potentially leading to restored memory and enhanced cognitive function.
What role do TIM-3 levels play in the cognitive function of Alzheimer’s patients?
In Alzheimer’s patients, elevated TIM-3 levels on microglia correlate with reduced ability to clear toxic amyloid beta plaques. This impaired function contributes to cognitive decline. Treatments targeting TIM-3 could potentially reverse this inhibition, resulting in improved cognitive function.
What potential does TIM-3 Alzheimer’s therapy offer in contrast to existing treatments?
TIM-3 Alzheimer’s therapy offers a promising alternative to existing treatments that primarily focus on amyloid beta. By specifically targeting the TIM-3 molecule, this therapy aims to enhance microglial clearance of plaques, addressing the underlying issues that contribute to Alzheimer’s-related cognitive decline.
How have studies demonstrated the effectiveness of TIM-3 therapy in animal models?
Studies demonstrated that mice with genetically altered TIM-3 levels exhibited enhanced plaque clearance and improvements in cognitive tasks, such as maze navigation. These findings suggest that inhibiting TIM-3 can restore some cognitive functions affected by Alzheimer’s.
What are the implications of TIM-3 Alzheimer’s therapy for future research?
The implications of TIM-3 Alzheimer’s therapy for future research include the potential development of anti-TIM-3 antibodies that can be tested in clinical settings. Understanding how TIM-3 manipulation affects microglial activity could lead to significant advancements in Alzheimer’s treatment strategies.
How does TIM-3 relate to the immune response in Alzheimer’s disease?
TIM-3 is a checkpoint molecule that modulates the immune response. In the context of Alzheimer’s disease, its overexpression on microglia prevents these cells from effectively attacking amyloid plaques. Hence, targeting TIM-3 offers a way to boost the brain’s natural immune response against Alzheimer’s pathology.
What is the next step in researching TIM-3 Alzheimer’s therapy?
The next step in researching TIM-3 Alzheimer’s therapy is to determine the efficacy of human anti-TIM-3 antibodies in mouse models of Alzheimer’s disease. This includes assessing whether targeting TIM-3 can prevent the formation of amyloid plaques and improve cognitive outcomes.
Are there any side effects associated with TIM-3 Alzheimer’s therapy?
While specific side effects of TIM-3 Alzheimer’s therapy have yet to be fully explored in clinical settings, any treatment aimed at modulating immune responses may carry potential risks. Ongoing research will focus on ensuring the safety and efficacy of targeting TIM-3 in Alzheimer’s therapy.
| Key Point | Details |
|---|---|
| Study Significance | The study explores the potential of using TIM-3 Alzheimer’s therapy, which has shown promising results in cancer treatment, for improving cognitive function in Alzheimer’s disease. |
| Research Focus | The research investigates the role of TIM-3, an immune checkpoint molecule that inhibits the action of microglia, brain immune cells that can clear Alzheimer’s plaques. |
| TIM-3 Function | TIM-3 serves to restrain immune cells, preventing them from overreacting, but in Alzheimer’s, it hinders the clearance of amyloid plaques. |
| Cognitive Improvement | Studies on mice lacking the TIM-3 gene show not only enhanced plaque clearance but also improved cognitive functions, like memory and behavior. |
| Future Steps | The next phase involves testing human anti-TIM-3 antibodies in mouse models to track plaque formation. |
Summary
TIM-3 Alzheimer’s therapy represents a revolutionary approach to combating cognitive decline associated with Alzheimer’s disease. This innovative method utilizes the role of TIM-3 checkpoint molecules, showing potential in reversing memory deficits and clearing harmful brain plaques. Research indicates that by inhibiting TIM-3, microglia can regain their function in Alzheimer’s models, leading to improved cognitive abilities. Future studies aim to evaluate the efficacy of TIM-3 inhibitors in human trials, potentially paving the way for novel therapeutic interventions against this debilitating condition.